From early development to industrial scale
As a globally leading contract development and manufacturing organization, we produce pharmaceutical ingredients and finished products as an integrated supplier.
A wide range of integrated services
Siegfried offers contract developing and manufacturing of active pharmaceutical ingredients, intermediates and finished dosage forms. Our integrated approach ensures that we can provide a wide range of services, from process development and optimization to registration, production, packaging and logistics, under a single roof.

Committed to deliver the most challenging projects
We are the strongest team dedicated to take our customers’ precious innovations to industrial scale. Across our global network, our team of experts is committed to do what ever it takes to ensure on-time-in-full delivery at the highest quality standards.

A global network to ensure security of supply
Siegfried counts on a global network of 13 facilities in seven countries on three continents for the production of active pharmaceutical ingredients and intermediates (Drug Substances) and finished dosage forms (Drug Products).
Expertise in process optimization for sustainable API production
With our expertise in process optimization, we help our customers to develop greener production processes, which require less energy and produce less waste, while maximizing the safety of our products. Siegfried is a member of the Dow Jones Sustainability Index Europe and rated AA by MSCI.

Strong quality track record
Siegfried stands out when it comes to exceptional quality and regulatory compliance. All Siegfried sites are focused on the highest cGMP standards and are approved by national and international authorities, including the U.S. Food and Drug Administration (FDA), Swissmedic, Japan’s Pharmaceuticals and Medical Devices Agency (PMDA), Spanish Agency of Medicines and Medical Devices (AEMPS), the German Health Authority and more.
Our additional services
integrated along the value chain
Project management
At Siegfried, we believe that effective project management goes far beyond finishing on time and within budget; it is about delivering top-notch services and taking care of our customers.
At the start of your project, we assign an experienced project manager and a team of experts from different fields, tailored to the project requirements. The project manager works closely with the customer to define the project’s scope. During execution, the project manager ensures timelines and budget, making adjustments as needed to handle any unexpected changes in close cooperation with the customer. The project manager also serves as the main point of contact for everyone involved and provides technical support for business development.
Our PMI based, well-defined and software supported project management approach ensures on-time-in-full delivery of all items and successful project execution. We are committed, easy to work with and are always endeavoring to reduce complexity.
Program management
Program management at Siegfried is the next step in a product’s life cycle. Once the project management team has successfully completed the validation of the process, program management takes over.
In program management, we look after the products that are strategic and important to both the client and Siegfried. Our well-experienced and well-skilled program managers will coordinate all technical product-related requirements, regardless if they are internal or external. This is done together and in close cooperation with the business manager who continues to look after the commercial aspects.
The program manager ensures on-time-in-full (OTIF) is reached on an individual campaign, but their area of responsibility goes far beyond a single campaign. Among the strategic aspects of a product, the program manager and their team of experts will assess future capacity requirements of a product, continuously explore improvements to further optimize process economics, and propose strategies to support the product growth.
Our goal is to support our customers with a dedicated team that takes care of all aspects of your commercial product.
Customer relationship management
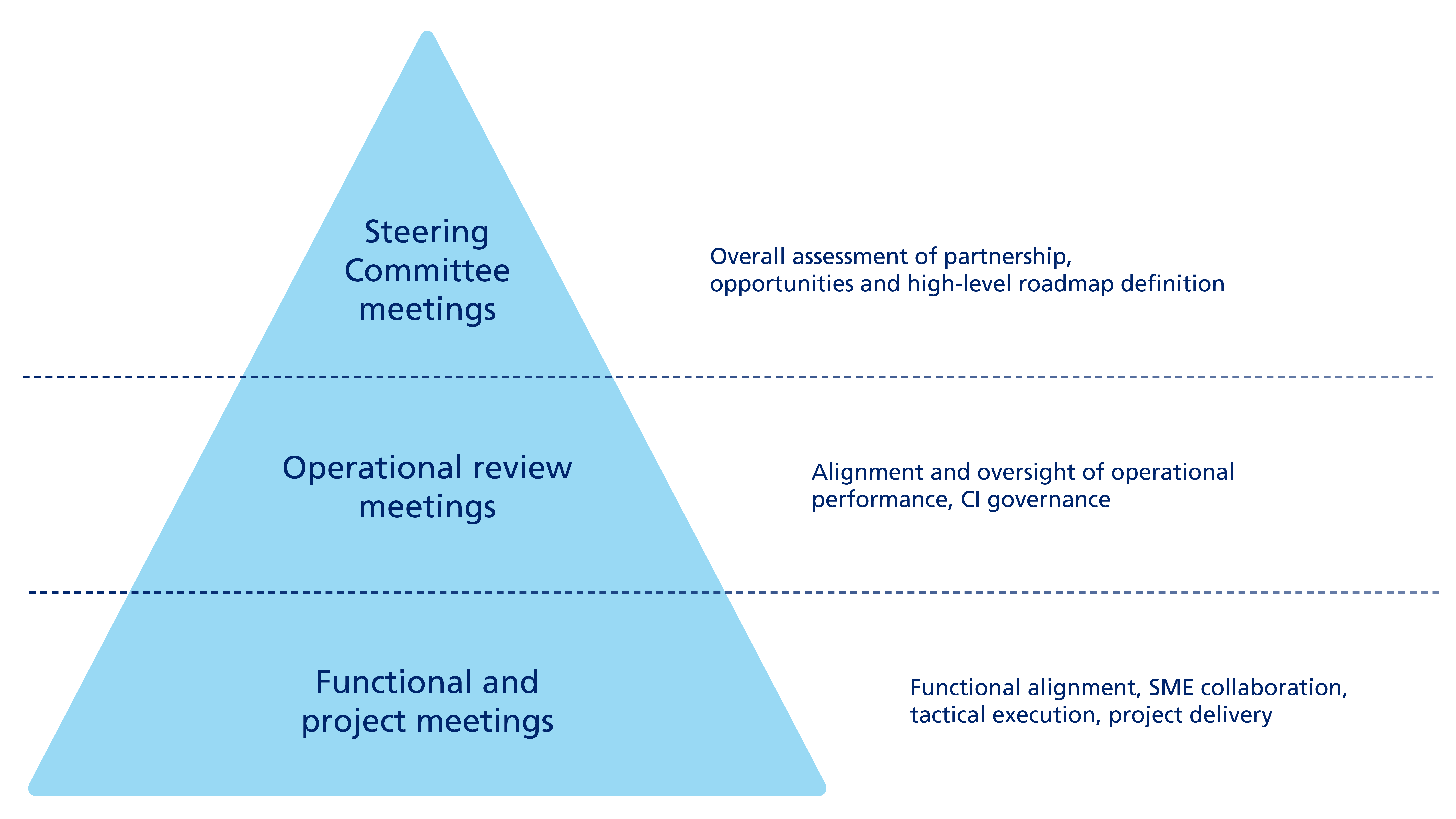
Siegfried sees communication as a key factor in being a trusted partner to the pharmaceutical industry. Siegfried maintains an ongoing and systematic dialogue with all customers. This dialogue is tailored to the needs of the individual customer and its organization, and depends on certain factors such as the strategic and/or commercial criticality of the business partnership, and includes regular discussions at both operational and strategic levels. As part of this dialogue, we measure and discuss customer satisfaction with our services at regular business review meetings.
Regulatory affairs
Our regulatory affairs team is crucial throughout your project. They not only ensure that active substances and medicinal products meet licensing and legal requirements but also ascertain product quality, safety and effectiveness in addition to compliance with laws and guidelines.
As an integrated supplier, we use our expertise in science, regulations, and business to ensure that our customers’ products meet global regulatory standards. We advise on and coordinate registration strategies of pharmaceuticals, devices and other products and lead or support marketing authorization applications from submission through response to health authority questions until final approval. Our regulatory affairs professionals are a crucial link between your company, your products and regulatory authorities, including the US Food and Drug Administration (FDA), the European Medicines Agency (EMA) and National Competent Authorities in Europe and other worldwide national regulatory bodies.
In addition to our consulting activity, we prepare and deliver the necessary approval documentation for active substances and medicinal products “ready-to-use” in eCTD or other formats to our customers; or as a DMF/CEP Holder submit directly to the corresponding authorities, respectively.
- Drug Master Files for the Europe, USA, Canada, China, Japan as well as other worldwide markets
- European Pharmacopoeia-Certificates of Suitability (CEP), Brazilian CADIFA
- Complete Quality (CMC) sections (Module 2.3 and 3) for marketing authorization applications
- Notifications of change
- Quality (CMC) documentation (Modules 3.2.S, 3.2.P) for IND or CTA submissions
- Briefing packages, or sections for Scientific Advices/Agency Meetings
We support our US customers with drug listing activities and amount reporting as per the CARES act. For the authorization of medicinal products in Europe, we are able to handle the complete regulatory process as an applicant.
Supply chain management
In pharmaceutical development and manufacturing, a reliable partner is crucial. We strive to build strong, long-lasting partnerships by offering customized supply chain solutions that meet our partners’ needs. Our commitment to excellence spans the entire value chain, ensuring a smooth transition between steps with equal attention paid to all.
To ensure a sustainable supply of materials and services, we select suppliers based on environmental and social responsibility criteria in addition to economic criteria. Our supply chain management involves coordinating multiple production sites, both within and outside the Siegfried network, using advanced planning tools and procurement principles to achieve the highest supply reliability.